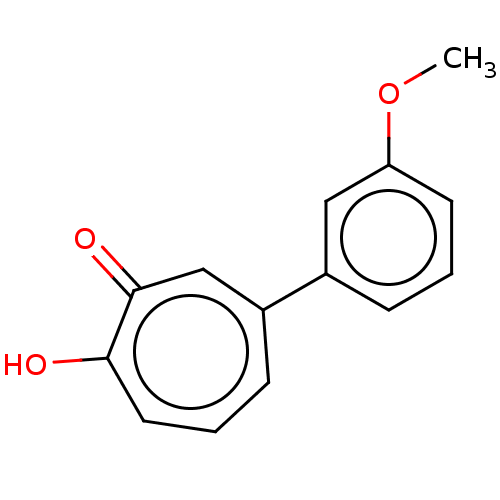
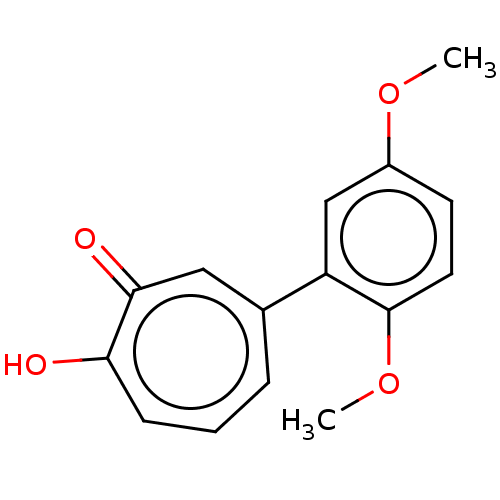
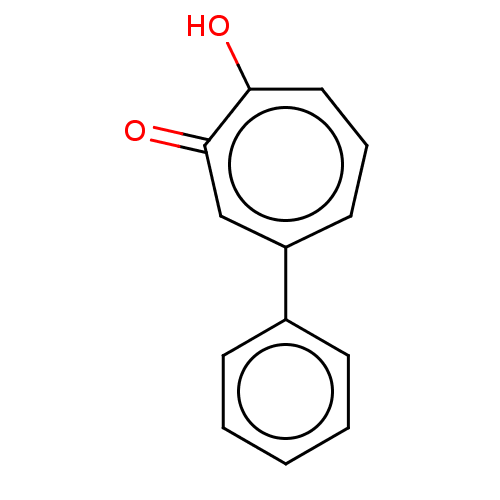
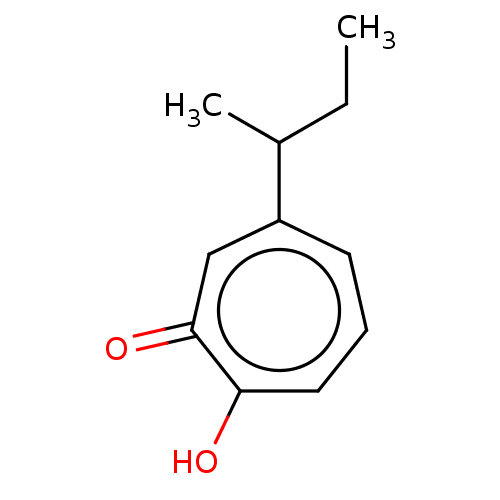
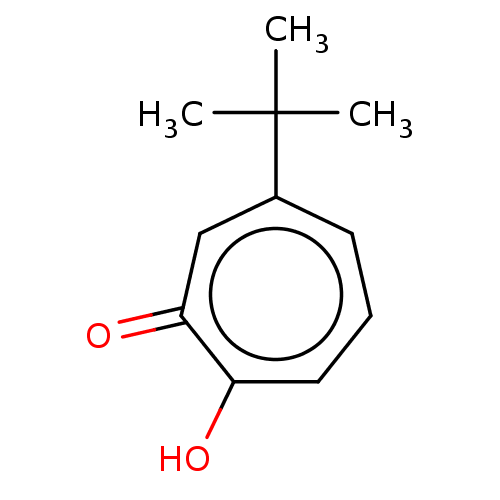
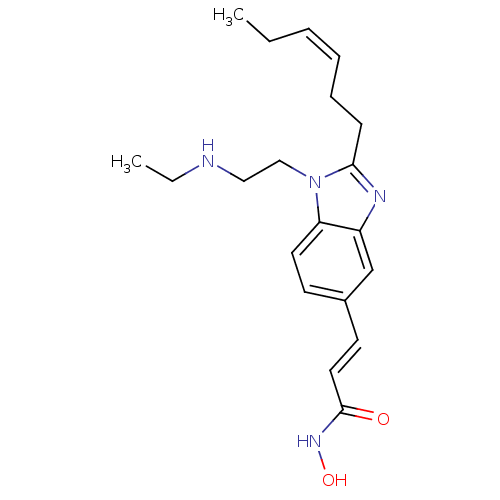
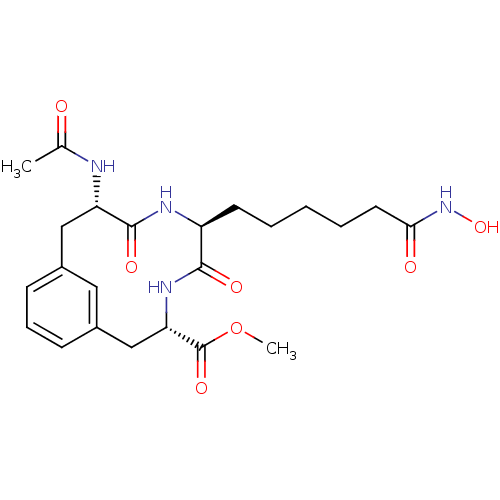
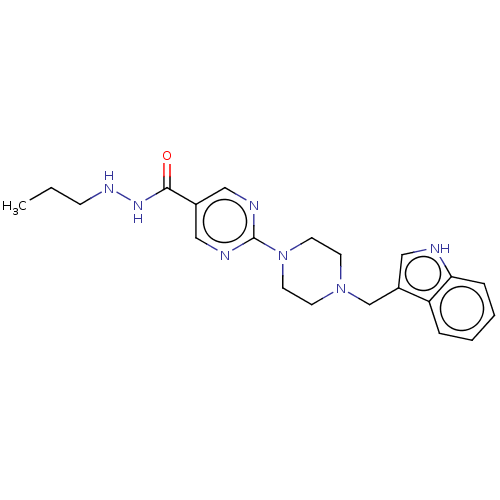
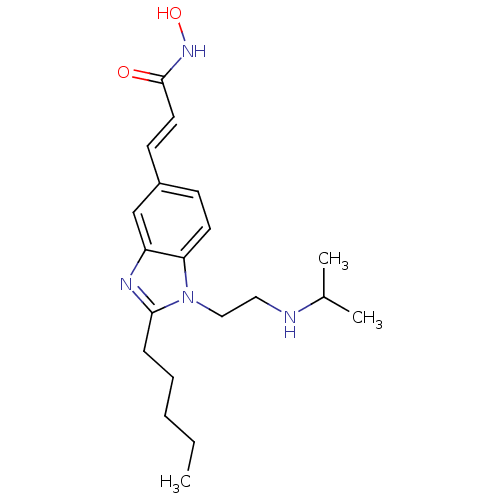
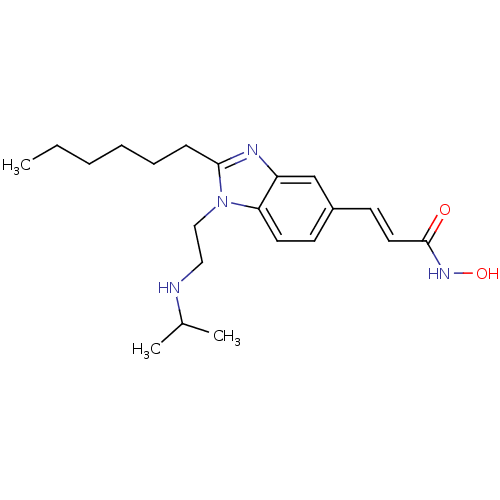
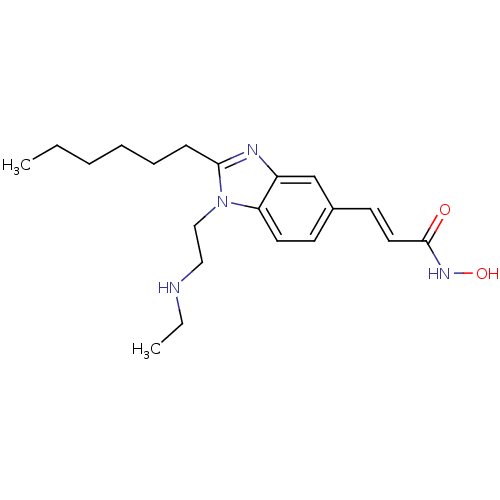
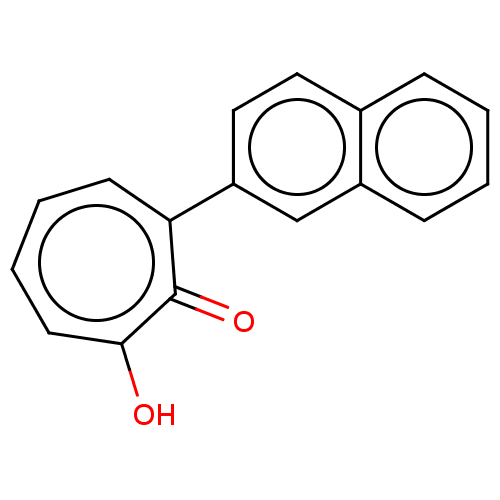
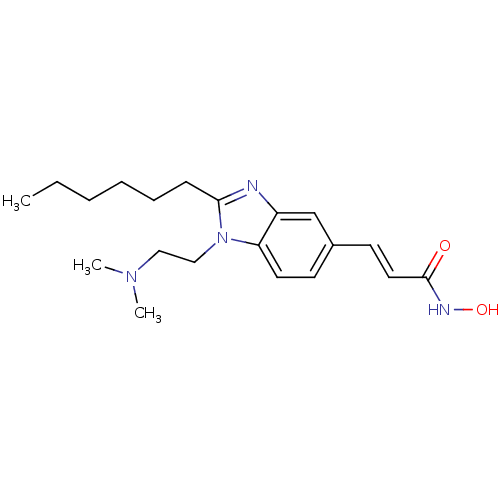
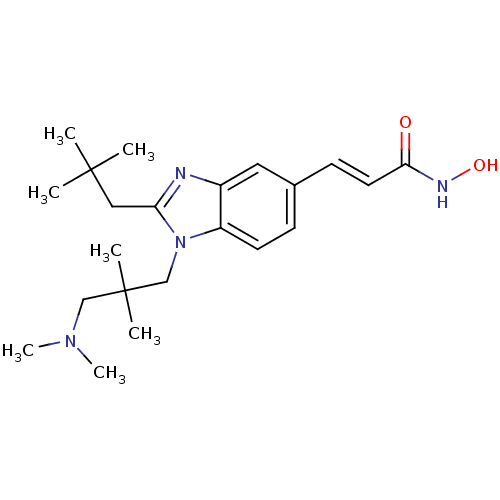
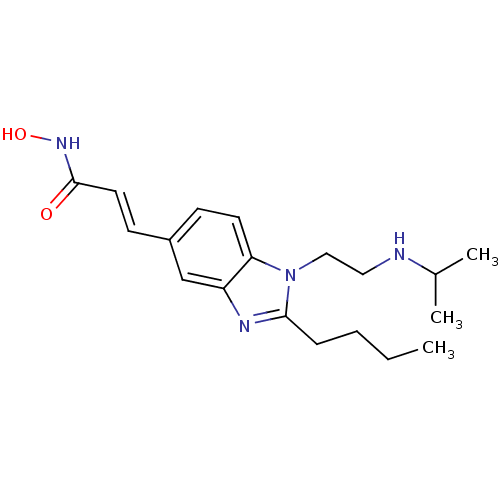
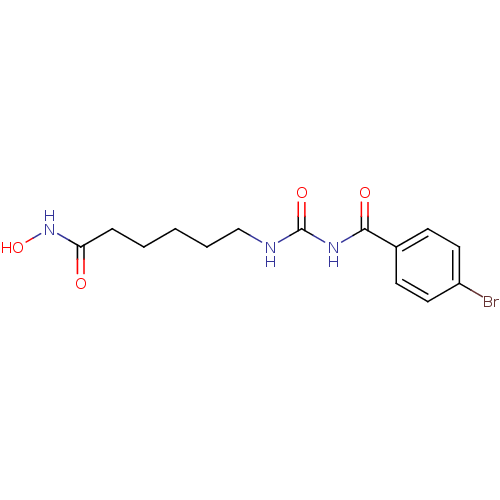
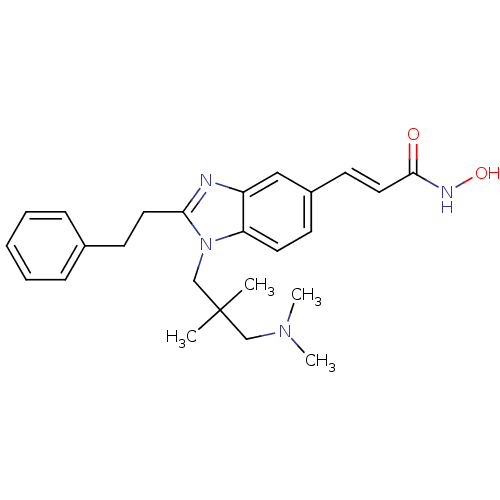
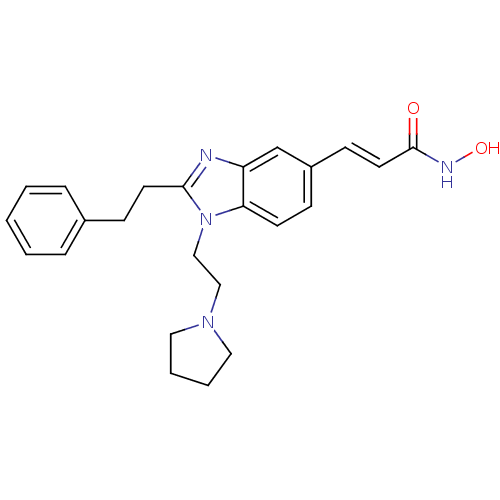
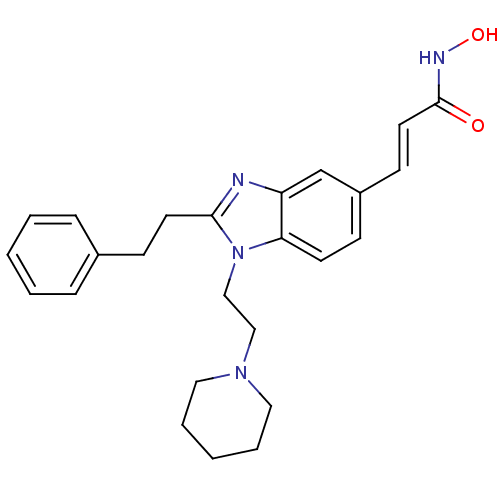
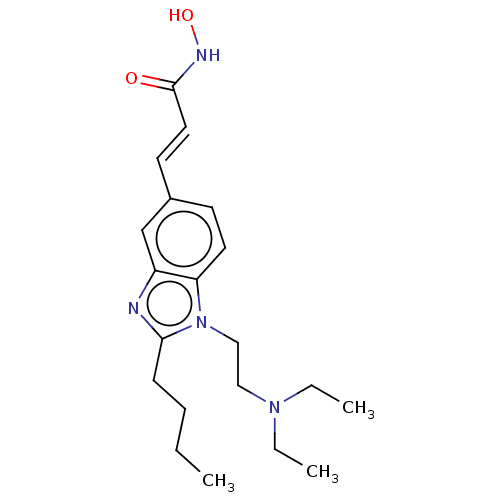
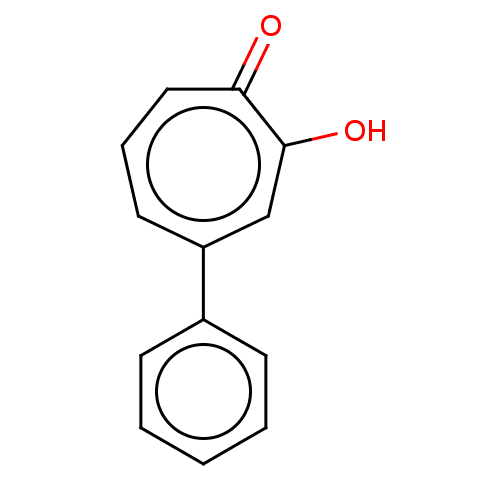
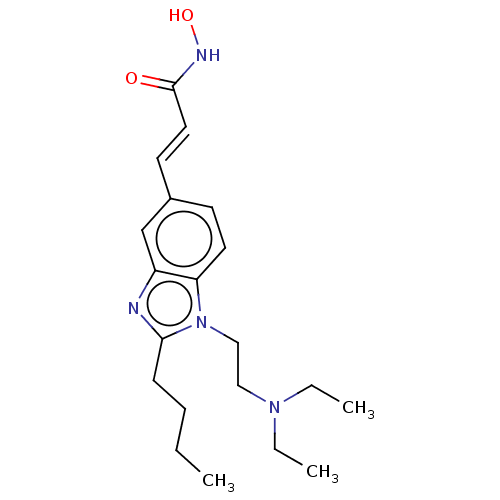
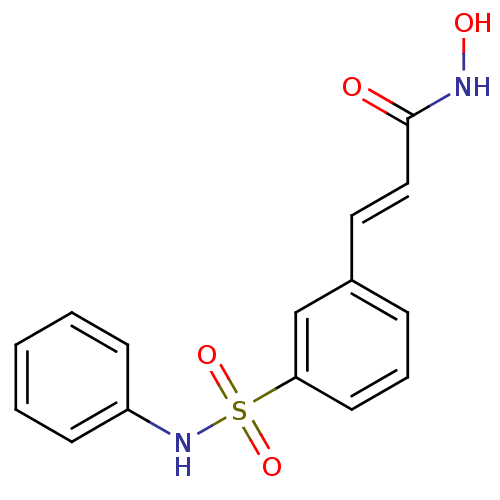
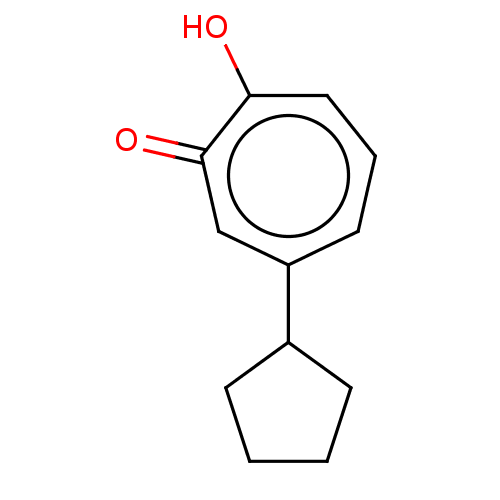
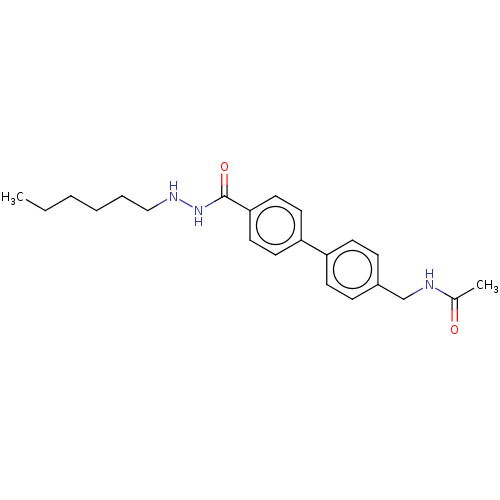
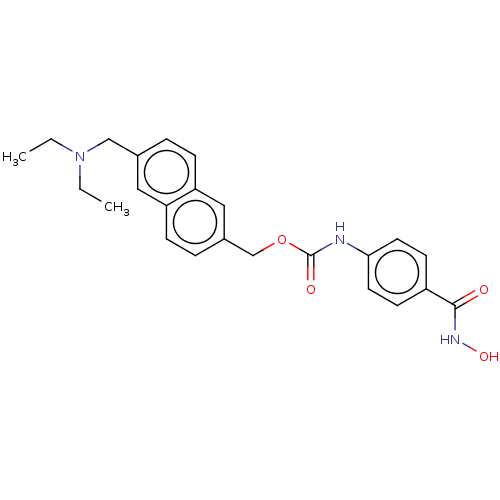
Affinity DataKi: 0.0400nMAssay Description:Key enzyme kinetic parameters for one class I HDAC (HDAC8) and one class Ha HDAC (HDAC4) were determined using commercially available human recombina...More data for this Ligand-Target Pair
Affinity DataKi: 0.100nMAssay Description:Inhibition of human HDAC8More data for this Ligand-Target Pair
Affinity DataKi: 0.140nMAssay Description:Key enzyme kinetic parameters for one class I HDAC (HDAC8) and one class Ha HDAC (HDAC4) were determined using commercially available human recombina...More data for this Ligand-Target Pair
Affinity DataKi: 0.170nMAssay Description:Key enzyme kinetic parameters for one class I HDAC (HDAC8) and one class Ha HDAC (HDAC4) were determined using commercially available human recombina...More data for this Ligand-Target Pair
Affinity DataKi: 0.440nMAssay Description:Key enzyme kinetic parameters for one class I HDAC (HDAC8) and one class Ha HDAC (HDAC4) were determined using commercially available human recombina...More data for this Ligand-Target Pair
Affinity DataKi: 0.5nMAssay Description:Inhibition of human HDAC8More data for this Ligand-Target Pair
Affinity DataKi: 0.530nMAssay Description:Competitive inhibition of human recombinant HDAC8 by Michaelis-Menten equation analysisMore data for this Ligand-Target Pair
Affinity DataKi: 0.700nMAssay Description:Competitive inhibition of HDAC5 using KI-104 as substrate by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataKi: 1.10nMAssay Description:Inhibition of human recombinant HDAC8 by Michaelis-Menten equation analysisMore data for this Ligand-Target Pair
Affinity DataKi: 1.36nMAssay Description:Key enzyme kinetic parameters for one class I HDAC (HDAC8) and one class Ha HDAC (HDAC4) were determined using commercially available human recombina...More data for this Ligand-Target Pair
Affinity DataKi: 1.42nMAssay Description:Key enzyme kinetic parameters for one class I HDAC (HDAC8) and one class Ha HDAC (HDAC4) were determined using commercially available human recombina...More data for this Ligand-Target Pair
Affinity DataKi: 1.5nMAssay Description:Inhibition of human recombinant HDAC8 by Michaelis-Menten equation analysisMore data for this Ligand-Target Pair
Affinity DataKi: 2.10nMAssay Description:Inhibition of human HDAC5More data for this Ligand-Target Pair
Affinity DataKi: 2.30nMAssay Description:Inhibition of human recombinant HDAC8 by Michaelis-Menten equation analysisMore data for this Ligand-Target Pair
Affinity DataKi: 2.40nMAssay Description:Inhibition of human recombinant HDAC8 by Michaelis-Menten equation analysisMore data for this Ligand-Target Pair
Affinity DataKi: 2.69nMAssay Description:Key enzyme kinetic parameters for one class I HDAC (HDAC8) and one class Ha HDAC (HDAC4) were determined using commercially available human recombina...More data for this Ligand-Target Pair
Affinity DataKi: 3.10nMAssay Description:Competitive inhibition of HDAC5 using KI-104 as substrate by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataKi: 4.30nMAssay Description:Inhibition of human recombinant HDAC5 using fluorogenic HDAC substrate class 2a after 30 mins by fluorimetrc methodMore data for this Ligand-Target Pair
Affinity DataKi: 5.32nMAssay Description:Inhibition of human HDAC8More data for this Ligand-Target Pair
Affinity DataKi: 5.60nMAssay Description:Competitive inhibition of HDAC5 using KI-104 as substrate by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataKi: 6.60nMAssay Description:Competitive inhibition of HDAC5 using KI-104 as substrate by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataKi: 6.80nMAssay Description:Competitive inhibition of HDAC5 using KI-104 as substrate by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataKi: 7.63nMAssay Description:Key enzyme kinetic parameters for one class I HDAC (HDAC8) and one class Ha HDAC (HDAC4) were determined using commercially available human recombina...More data for this Ligand-Target Pair
Affinity DataKi: 7.90nMAssay Description:Inhibition of human recombinant HDAC8 by Michaelis-Menten equation analysisMore data for this Ligand-Target Pair
Affinity DataKi: 9.20nMAssay Description:Competitive inhibition of HDAC5 using KI-104 as substrate by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataKi: 10.1nMAssay Description:Key enzyme kinetic parameters for one class I HDAC (HDAC8) and one class Ha HDAC (HDAC4) were determined using commercially available human recombina...More data for this Ligand-Target Pair
Affinity DataKi: 11nMAssay Description:Competitive inhibition of HDAC5 using KI-104 as substrate by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataKi: 11nMAssay Description:Competitive inhibition of HDAC5 using KI-104 as substrate by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:Inhibition of human recombinant HDAC8 by Michaelis-Menten equation analysisMore data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:Inhibition of recombinant human C-terminal His-tagged HDAC8 (1 to 377 residues) expressed in baculovirus-infected insect cells using RHK(Ac)K(Ac)AMC ...More data for this Ligand-Target Pair
Affinity DataKi: 16nMAssay Description:Competitive inhibition of HDAC5 using KI-104 as substrate by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataKi: 17nMAssay Description:Competitive inhibition of HDAC5 using KI-104 as substrate by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataKi: 17nMAssay Description:Inhibition of recombinant HDAC5 by fluorimetric assayMore data for this Ligand-Target Pair
Affinity DataKi: 18nMAssay Description:Competitive inhibition of HDAC5 using KI-104 as substrate by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataKi: 19nMAssay Description:Competitive inhibition of HDAC8 using KI-104 as substrate by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataKi: 19.4nMAssay Description:Key enzyme kinetic parameters for one class I HDAC (HDAC8) and one class Ha HDAC (HDAC4) were determined using commercially available human recombina...More data for this Ligand-Target Pair
Affinity DataKi: 21nMAssay Description:Inhibition of human HDAC5More data for this Ligand-Target Pair
Affinity DataKi: 21nMAssay Description:Competitive inhibition of HDAC5 using KI-104 as substrate by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataKi: 22nMAssay Description:Competitive inhibition of HDAC8 using KI-104 as substrate by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataKi: 22nMAssay Description:Competitive inhibition of HDAC8 using KI-104 as substrate by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataKi: 23.3nMAssay Description:Key enzyme kinetic parameters for one class I HDAC (HDAC8) and one class Ha HDAC (HDAC4) were determined using commercially available human recombina...More data for this Ligand-Target Pair
Affinity DataKi: 24nMAssay Description:Inhibition of human recombinant HDAC8 using fluorogenic HDAC substrate after 15 mins by fluorimetrc methodMore data for this Ligand-Target Pair
Affinity DataKi: 25nMAssay Description:Inhibition of human HDAC8More data for this Ligand-Target Pair
Affinity DataKi: 25nMAssay Description:Competitive inhibition of HDAC5 using KI-104 as substrate by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataKi: 36nMAssay Description:Competitive inhibition of HDAC5 using KI-104 as substrate by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataKi: 39nMAssay Description:Inhibition of human HDAC8More data for this Ligand-Target Pair

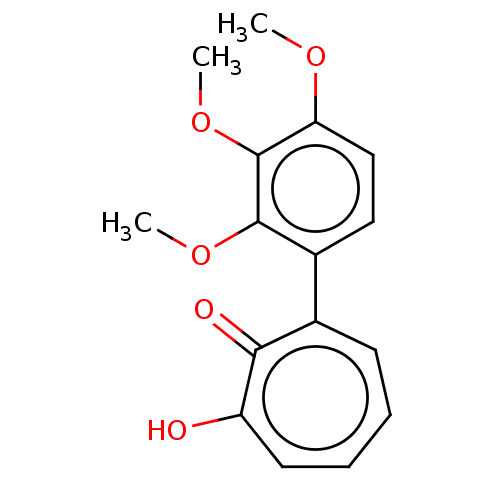
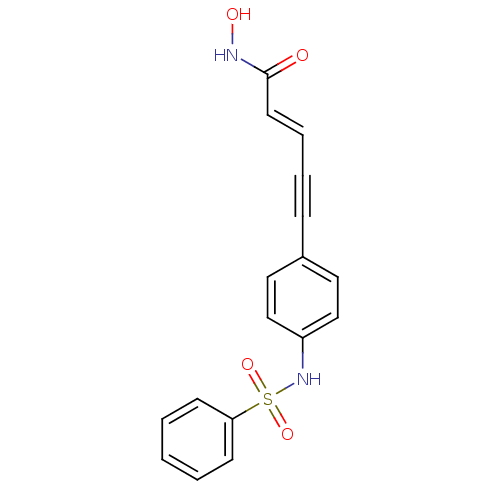
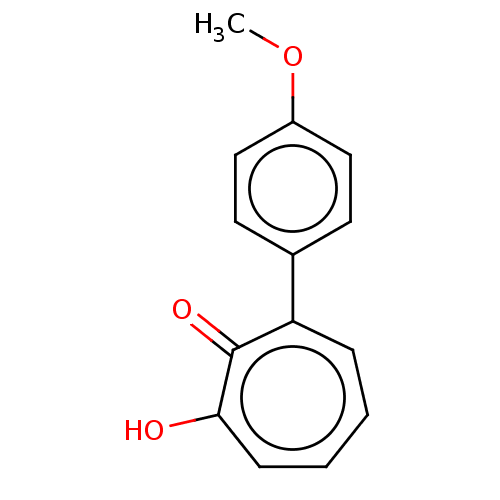
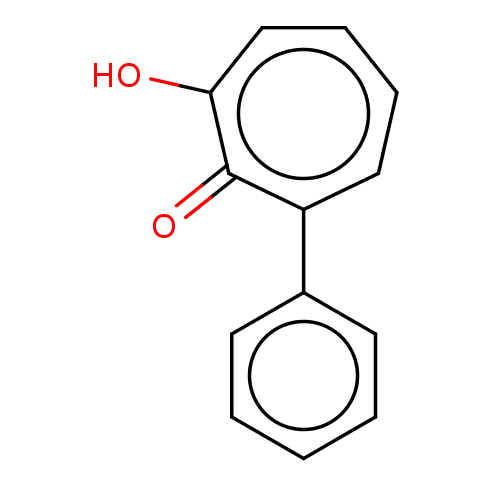
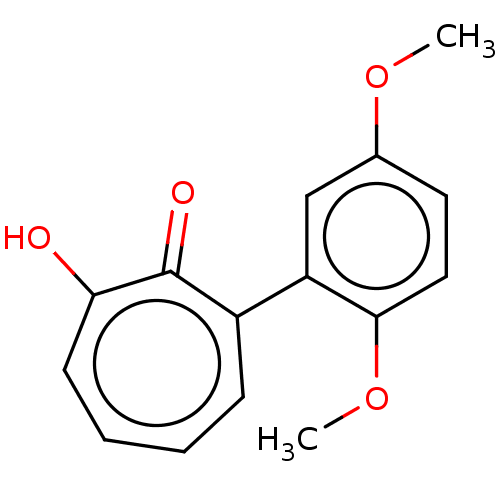

 3D Structure (crystal)
3D Structure (crystal)